Abstract
Treatment of acute myeloid leukemia (AML) has been enhanced by the development and regulatory approval of a series of novel agents, including midostaurin and gilteritinib (FLT3 inhibitors), venetoclax (BCL2 inhibitor), ivosidenib (IDH1 inhibitor), and enasidenib (IDH2 inhibitor). A difficulty that has arisen in the era of molecular therapies, however, is determining the efficacy of these agents for patients with AML harboring atypical driver mutations. These atypical drivers were underrepresented in seminal clinical trials that led to the approval of targeted AML therapies, thereby limiting the availability of data for clinical decision making.
For example, the non-canonical FLT3 p.N676K variant was initially described as an acquired resistance mechanism in patients with FLT3 internal tandem duplication (ITD) mutations who were treated with midostaurin. Follow-up studies in Ba/F3 cells demonstrated that the FLT3 N676K mutation is sensitive to midostaurin and quizartinib, but that co-occurrence of FLT3 N676K and ITD mutations confers resistance to both targeted agents. Clinical data from patients with the FLT3 N676K mutation, however, is limited to reports of two patients, both of whom developed the N676K mutation at relapse. There have been no published reports detailing the treatment of patients with the FLT3 N676K mutation with de novo disease, and limited data exist regarding the role of FLT3 inhibition for patients with the FLT3 N676K mutation in the clinical setting.
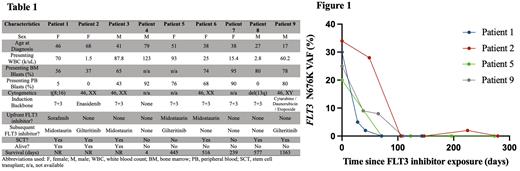
To address this knowledge gap, we detail our experience caring for nine individuals with AML harboring FLT3 N676K at The University of Chicago. The patients were identified by querying the University of Chicago molecular pathology database as part of an IRB-approved retrospective analysis. Six of these patients had AML with FLT3 N676K arising as the only FLT3 variant, whereas the mutational composition of three patients carried coincident ITD or tyrosine kinase domain (TKD) mutations at some point during their clinical course. The median age at diagnosis of AML in our cohort was 41 years, with a mean presenting WBC count of 53.3 k/uL. Four of nine patients had normal cytogenetics. Additional demographic and disease-specific parameters are depicted in Table 1.
One elderly patient pursued comfort care immediately after presentation. Of the remaining eight patients, six received FLT3 inhibitors at any point during their clinical trajectory. Three patients were treated with FLT3 inhibitors added to upfront standard induction therapy (midostaurin, n=2 ; sorafenib, n=1). Of those, one (patient #1) achieved a morphologic and molecular complete remission and proceeded to curative-intent allogeneic transplant. The remaining two patients (patients #5 and #6) harbored co-occuring FLT3 mutations, one with FLT3 ITD and the other with FLT3 TKD, and both experienced relapse after an initial complete remission following induction therapy. In patients for whom longitudinal next-generation sequencing data from bone marrow specimens was available, we observed dramatic reduction in measurable FLT3 N676K variant allele frequencies after FLT3 inhibitor use, regardless of line of therapy (Fig. 1).
Here, we describe the largest series to date of AML patients with atypical FLT3 N676K driver mutations. Affected individuals in our cohort presented at a notably younger age and demonstrated reductions in disease burden with the use of targeted FLT3 inhibitors. The presence of concurrent canonical FLT3 mutations was associated with loss of treatment response. Longer-term follow will help evaluate the durability of FLT3 inhibitor maintenance strategies in both transplant and non-transplant clinical settings.
Disclosures
Artz:Abbvie: Honoraria; Magenta: Honoraria. Stock:Pluristem: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; MorphoSys: Honoraria; Kura Oncology: Honoraria; Kite: Honoraria; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; Servier: Honoraria; Syndax: Consultancy, Honoraria; Newave Pharmaceuticals: Consultancy; Agios: Honoraria. Odenike:Abbvie; Impact Biomedicines; Celgene; Novartis; BMS; Taiho Pharmaceutical; CTI; Threadwell therapeutics; Bristol-Myers Squibb/Celgene (Inst): Consultancy; Celgene (Inst); Incyte (Inst); Astex Pharmaceuticals (Inst); NS Pharma (Inst); Abbvie (Inst); Janssen Oncology (Inst); OncoTherapy Science (Inst); Agios (Inst); AstraZeneca (Inst); CTI BioPharma Corp (Inst); Kartos Therapeutics (Inst); Aprea AB (Inst): Research Funding. Dworkin:Abbvie: Honoraria. Wool:Diagnostica Stago: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal